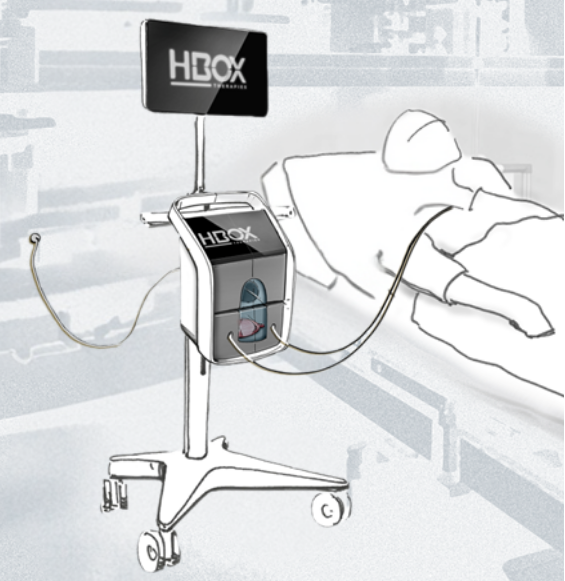
Acute Respiratory Failure (ARF) is one of the leading causes of death worldwide and a daily reality in intensive care units [2]. It occurs when the lungs can no longer exchange enough oxygen and/or carbon dioxide, often due to conditions like pneumonia, COPD, ARDS, or respiratory viruses.
The current standard of care, invasive mechanical ventilation (IMV), frequently leads to serious complications including lung injury, muscle degradation, and infection, with a high mortality rate of over 35%[3]. Patients are typically sedated, which limits communication, increases ICU burden, and prolongs recovery.
Despite its widespread use, IMV remains a high-risk, resource-intensive therapy. A safer, more sustainable alternative is urgently needed to improve outcomes and reduce the strain on patients, clinicians, and healthcare systems [4].
1. Jaber, S., Citerio, G. & Slutsky, A.S. Acute respiratory failure and mechanical ventilation in the context of the COVID-19 pandemic: why a special issue in ICM?. Intensive Care Med 46, 2131–2132 (2020). https://doi.org/10.1007/s00134-020-06298-7
2. WHO (https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death)
3. Welker, C. et. al. 2021, 2021 Acute Respiratory Distress Syndrome Update, With Coronavirus Disease 2019 Focus, Journal of Cardiothoracic and Vascular Anesthesia, Volume 36, Issue 4, 1188 - 1195. https://doi.org/10.1053/j.jvca.2021.02.053
4. Extracorporeal Life Support: The ELSO Red Book, 6th Edition